FRONTIERS IN MEDICAL CASE REPORTS - Volume 6; Issue 4, (Jul-Aug, 2025)
Pages: 01-05
Print Article
Download XML Download PDF
SISCOM Findings in Atonic Seizure Due To Lennox–Gastaut Syndrome
Author: Darío Lisei, Álvaro Baena, Nuria Bargalló, Aida Niñerola-Baizan Engineer, Katherine Quintero, Antonio Donaire, Xavier Setoain
Category: Medical Case Reports
Abstract:
The present case documents the 99mTc-HMPAO subtraction ictal SPECT and co-registered with MRI (SISCOM) high uptake in left thalamus, right caudate nucleus, left frontal and superior temporal gyrus and brainstem in a Lennox–Gastaut Syndrome (LGS) patient, supporting the notion that LGS is not solely a cortical disorder but rather involves largescale thalamocortical and striato brainstem networks.
Keywords: SISCOM, Brain SPECT, Brain PET/CT, Atonic seizure, Lennox-Gastaut Syndrome
Full Text:
Introduction
Lennox-Gastaut Syndrome (LGS) is a lifelong, developmental epileptic encephalopathy characterized by frequent seizures, including atonic, tonic, and atypical absence seizures, which contribute to behavioral disorders and intellectual disabilities (Amuktar and Lui, 2025). The exact pathophysiology underlying LGS remains unclear. EEG, functional MRI, and deep brain stimulation studies suggest that the thalamus and pons play a crucial role in facilitating seizure activity in LGS (Poulen et al., 2025). The present case shows the cortical and subcortical involvement network in a patient with LGS documented by an early injection SISCOM analysis.
Case
A 21-year-old girl with past medical history of idiopathic psychomotor developmental delay. At the age of 20 years old she had her first generalized tonic-clonic seizure. MRI was normal and EEG showed multifocal/bisynchronous discharges and a pattern more consistent with LGS. 18F-FDG brain PET/CT showed a mild to moderate bilateral frontal hypometabolism and SISCOM was notable for markedly uptake in left thalamus, right caudate nucleus, left frontal and superior temporal gyrus and brainstem.
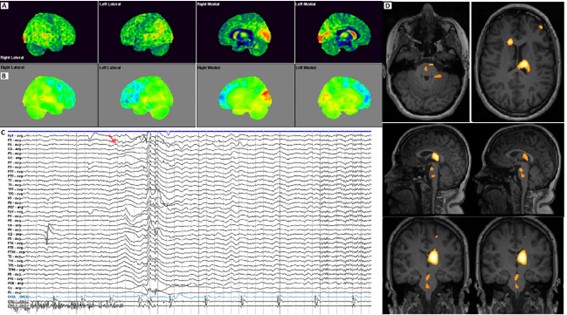
Figure 1
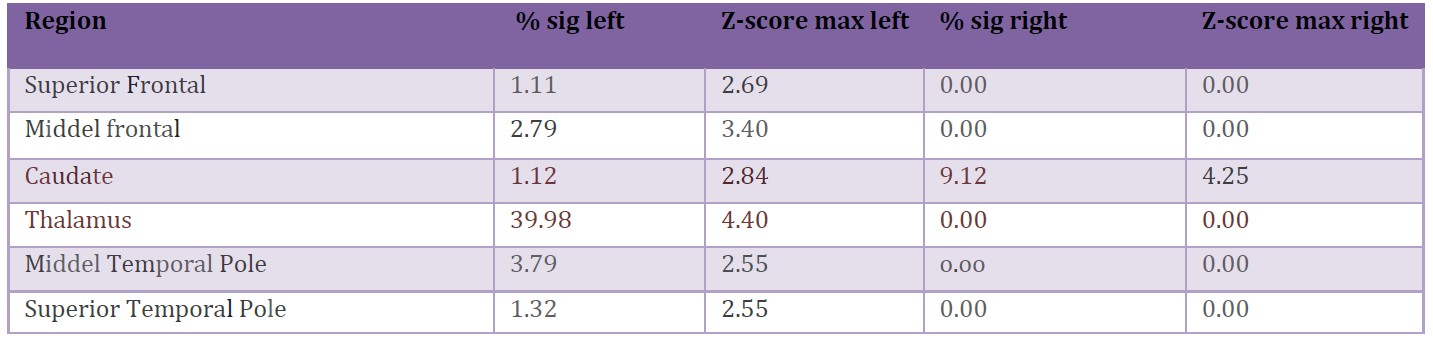
(Fig. 1) 18F-FDG PET/CT (A, PET cortical surface normalized; B, PET cortical Surface parametrical maps) both bilateral left predominant decreased radiotracer uptake in medial and lateral association frontal cortex. EEG (C, representative EEG epoch illustrating the onset of a typical atonic seizure in this patient. The record is displayed over 10 seconds/page in a referential montage with a common-average reference (International 10–20 system), and supplementary frontotemporal electrodes. High-and low-frequency filter settings were 30 Hz and 1 Hz, respectively. EMG leads (bottom two channels) were placed over both deltoids to capture muscle activity. The red arrow indicates the moment of seizure onset, characterized by a generalized electro decrement accompanied by a high-voltage delta slow wave over the right hemisphere, followed by generalized delta activity most prominent in the frontotemporal regions. Superimposed on this slow activity, a low-voltage, fast (beta) rhythm emerges toward the end of the page. Notably, from the onset of the seizure, EMG activity markedly diminishes, consistent with the sudden loss of muscle tone characteristic of an atonic seizure. Ictal SPECT injection was performed in this atonic seizure, 4 seconds after the onset in a seizure of 15 seconds. SISCOM images using Neurocloud® Software using AAL Atlas age-matched normal controls (D, Z-scores superimposed on MRI) revealed a markedly high uptake in left thalamus (medial region), right head caudate nucleus, and mild to moderate in left middle frontal gyrus, left superior temporal gyrus (Table 1), and additionally in brainstem areas related to ascending reticular activating system (ARAS - interpeduncular nucleus, nucleus reticularis pontis caudalis with anterior and left extension).
Table 1: ROI Atlas Analysis (AAL Atlas).
Patients with this type of epilepsy may have multiple types of seizures: absence, atonic, tonic and tonic-clonic. In these cases, the epileptic activity itself can contribute to the development of cognitive-behavioral disorders. Although morphometric and connectivity alterations of the thalamus have been extensively documented in idiopathic generalized epilepsy (Wang et al., 2012), subcortical networks—particularly the thalamus—are also central to the pathophysiology of Lennox–Gastaut Syndrome (LGS). In LGS, the centromedian nucleus (CM) and other intralaminar nuclei frequently show electrophysiological involvement, especially during atonic seizures (Velasco et al., 2000). Our patient’s SISCOM images demonstrated notable hyperperfusion in the left thalamus, right caudate nucleus, and upper brainstem, regions known to participate in the generation and modulation of generalized epileptiform activity (Velasco et al., 2006).
Atonic seizures—often characterized by abrupt loss of muscle tone—may be explained by pathological activation or dysfunction within these subcortical circuits. The reticulothalamic pathways, particularly, have been implicated in “generalized” seizure propagation by way of reverberating loops through the ascending reticular activating system (ARAS). The prominent subcortical hyperperfusion in this patient’s SISCOM study supports the notion that LGS is not solely a cortical disorder but rather involves large?scale thalamocortical and striato?brainstem networks. Further research into the functional imaging of subcortical structures in LGS could help elucidate novel targets for therapy—such as deep?brain stimulation of the centromedian thalamic nucleus—to address the often?intractable nature of these seizures (Zillgitt et al., 2022).
Conclusion
Our patient’s SISCOM images demonstrated notable hyperperfusion in the left thalamus, right caudate nucleus, and upper brainstem regions, known to participate in the generation and modulation of generalized epileptiform activity, supporting the notion that LGS is not solely a cortical disorder but rather involves large?scale thalamocortical and striato?brainstem networks. Further research into the functional imaging of subcortical structures in LGS could help elucidate novel targets for therapy—such as deep?brain stimulation— to address the often?intractable nature of these seizures.
Conflict of Interest: Authors declare no conflict of interest.
Author Contributions: DL, AB, XS and AD literature search and review. All authors: Manuscript writing and editing.
Compliance with Ethical Standards: this article does not contain any studies with human or animal subjects performed by the any of the authors.
Consent: Written consent was obtained.
References:
Amuktar CV, Lui F. Lennox-Gastaut Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan.
Poulen G, Gélisse P, Crespel A, Chan-Seng E, Moser PO, Coubes P. Does deep brain stimulation of the anterior nucleus of the thalamus represent the future of Lennox-Gastaut syndrome? J Neurol 2025; 272: 312.
Velasco AL, Velasco F, Jiménez F, Velasco M, Castro G, Carrillo-Ruiz JD, Fanghänel G, Boleaga B. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia 2006; 47: 1203-1212.
Velasco F, Velasco AL, Velasco M. Centromedian thalamic stimulation in intractable epilepsy: I. Clinical, electroencephalographic, and behavioral observations. Epilepsia 2000; 41: S20-S29.
Wang Z, Zhang Z, Jiao Q, Liao W, Chen G, Sun K, Shen L, Wang M, Li K, Liu Y, Lu G. Impairments of thalamic nuclei in idiopathic generalized epilepsy revealed by a study combining morphological and functional connectivity MRI. PLoS One 2012; 7: e39701.
Zillgitt AJ, Haykal MA, Chehab A, Staudt MD. Centromedian thalamic neuromodulation for the treatment of idiopathic generalized epilepsy. Front Hum Neurosci 2022; 16: 907716.
|


