FRONTIERS IN MEDICAL CASE REPORTS - Volume 6; Issue 4, (Jul-Aug, 2025)
Pages: 01-08
Print Article
Download XML Download PDF
Respiratory Colonization or Infection? Unraveling The Origin of Methicillin-Sensitive Staphylococcus Aureus Bacteremia in An Immunosuppressed Patient with Systemic Lupus Erythematosus
Author: Ling Qin, Shiyu Chen, Lina Guo, Chao Li, Ying Ge
Category: Medical Case Reports
Abstract:
This case report describes the clinical course of methicillin-sensitive Staphylococcus Aureus (MSSA) bacteremia in a 44- year old female patient with systemic lupus erythematosus (SLE) under immunosuppressive therapy. The patient was initially hospitalized due to active SLE. After the disease activity was controlled, she developed fever and hypoxemia. Blood cultures and lower respiratory tract secretions both yielded MSSA. Whole-genome sequencing revealed that the bloodstream and respiratory isolates were clonally related. Although chest imaging showed no typical signs of bacterial pneumonia, a comprehensive evaluation found no evidence of MSSA infection in other potential sites, such as the urinary tract or skin and soft tissues. This supports the hypothesis that disruption of the alveolar-capillary barrier may have facilitated the translocation of respiratory tract bacteria into the bloodstream, leading to bacteremia. The respiratory tract is thus considered a potential source of infection. This case highlights the need to remain vigilant for bacteremia caused by pulmonary barrier disruption in immunosuppressed patients, even in the absence of classical imaging features of MSSA pneumonia, and underscores the potential value of genomic epidemiology in infection source tracing.
Keywords: Methicillin Susceptible Staphylococcus Aureus, Systemic Lupus Erythematosus, Bacteremia, Immunosuppression
Full Text:
Introduction
Infection continues to be a leading cause of morbidity and mortality in systemic lupus erythematosus (SLE) (Xuan et al., 2025). Among these infections, bacteremia represents a particularly severe complication, often occurring during active disease phases and in patients exposed to high-dose corticosteroids or prolonged immunosuppressive therapy (Kim et al., 2023).
Previous studies have demonstrated that bacteremia in SLE is frequently associated with severe disease flares, immunosuppressive drug exposure, and systemic comorbidities. Importantly, disease-related organ damage itself has been identified as a critical predisposing factor for bacteremia, likely due to persistent immune dysregulation, tissue barrier dysfunction, and increased susceptibility to opportunistic pathogens (Rúa-Figueroa et al., 2020).
The prognosis of SLE patients with bacteremia remains poor, with high recurrence rates and significant mortality (Wang et al., 2020), underscoring the need for early recognition and aggressive management. Here, we report a rare case of methicillin-sensitive Staphylococcus Aureus (MSSA) bacteremia in an immunosuppressed SLE patient, where the respiratory tract was identified as the probable infection source despite lacking typical pneumonia features. Whole-genome sequencing confirmed the clonal identity of MSSA isolated from both blood and respiratory secretions, suggesting hematogenous spread facilitated by SLE-related pulmonary structural damage rather than primary pulmonary infection. This case highlights how SLE-associated tissue damage may act as a nidus for bacteremia, emphasizing the need for clinicians to maintain a high index of suspicion even in the absence of overt infection signs.
Case Presentation
A 44-year-old Asian woman with longstanding diabetes mellitus and lupus nephritis (diagnosed in 2011) presented in December 2024 with a three-month history of persistent fever, generalised rash, edema, nausea, non-projectile vomiting (2–3 episodes daily), and periumbilical pain. Her immunosuppressive regimen included sequential corticosteroid/cyclophosphamide therapy followed by cyclosporine A (CSA), which had previously achieved disease stabilization. Physical examination revealed conjunctival pallor, bilateral lower limb edema, and a soft abdomen with mild deep tenderness in the epigastric/left lower quadrants though negative Murphy's sign and normoactive bowel sounds (4/minute). Vital signs remained stable throughout admission, with intermittent febrile episodes peaking at 38.2 °C.
Results of Laboratory Tests and Imaging Examinations
Laboratory tests showed leukopenia (WBC 2.09×10?/L), anemia (Hb 57 g/L), thrombocytopenia (PLT 21×10?/L), and nephrotic-range proteinuria (urinary protein ?3.0 g/L, RBC 277.5/µL, abnormal RBC percentage 60%, 24-hour urinary protein 2.50 g/1500 mL). Blood chemistry revealed hypoalbuminemia (Alb 14 g/L), normal liver enzymes and renal function. Complement levels were significantly reduced (C3 0.168 g/L, C4 0.007 g/L), with elevated anti-dsDNA IgG (1207.7 IU/mL) and positive direct Coombs' test. Infectious workup, including blood cultures, plasma CMV and EBV-DNA, and peripheral monocyte T-SPOT.TB, were negative. Chest CT (Fig. 1A) showed minor patchy infiltrates in the lower lungs, while abdominal ultrasound revealed gallbladder wall thickening with normal intestinal findings.
The SLE Disease Activity Index (SLEDAI) was 16, indicating severe disease activity. Methylprednisolone (MP) 500 mg daily for three days was initiated, followed by MP 80 mg daily for five days. By day 3, fever and digestive symptoms resolved, appetite returned, and bowel movements normalized. The hematologic abnormalities, and complement levels improved. However, on day 5, she redeveloped fever (Tmax 38.5°C), and by week 1, she developed hypoxemia, orthopnea, and hemoptysis (fresh bloody sputum). Examination revealed RR 30 bpm, SpO? 79% (RA) and bilateral lower lung crackles. BNP was elevated at 2190 ng/mL, and chest CT on day 7 (Fig. 1B) showed new diffuse peri-bronchovascular infiltrates. Left heart failure was suspected, and diuretics were administered. Meanwhile due to relapsed fever, empirical vancomycin and ceftazidime were initiated and MP was gradually adjusted to 40 mg once daily. There was no clinical evidence of skin and soft tissue infection, and both abdominal symptoms, urine bacteria culture and physical examination were normal. However, both blood and sputum from lower respiratory tract cultures identified MSSA via MALDI-TOF, leading to targeted therapy with amoxicillin-clavulanate for two weeks of whole antibiotic therapy. One week later, dyspnea resolved, hemoptysis ceased, SpO? normalized (97% RA), BNP decreased to 416 ng/ml and the infiltrates on chest CT disappeared (on day 14, Fig. 1C). Due to dynamic changes in imaging within a short timeframe, and diffuse bilateral ground-glass opacity, this strongly supports pulmonary edema over MSSA pneumonia itself, nor was it pulmonary involvement due to lupus activity.
Pathogen Homology Analysis
In order to identify the origin of MSSA, the whole-genome sequencing of four MSSA isolates (blood: 24B39558, 24B39703; sputum: 24R19231, 24R19348) showed >95% average nucleotide identity (ANI) with reference strain NCTC8325, confirming S. aureus (Richter and Rosselló-Móra, 2009). Multilocus sequence typing (MLST) identified all isolates as ST5 with 0–5 core genome SNP differences, indicating clonal transmission (Figure 2) (Schürch et al., 2018; Jolley and Maiden, 2010). Phylogenetic analysis with 168 Beijing S. aureus strains from NCBI GenBank confirmed that bloodstream and respiratory isolates clustered together, suggesting hematogenous infection potentially originating from respiratory colonization (Fig. 2).
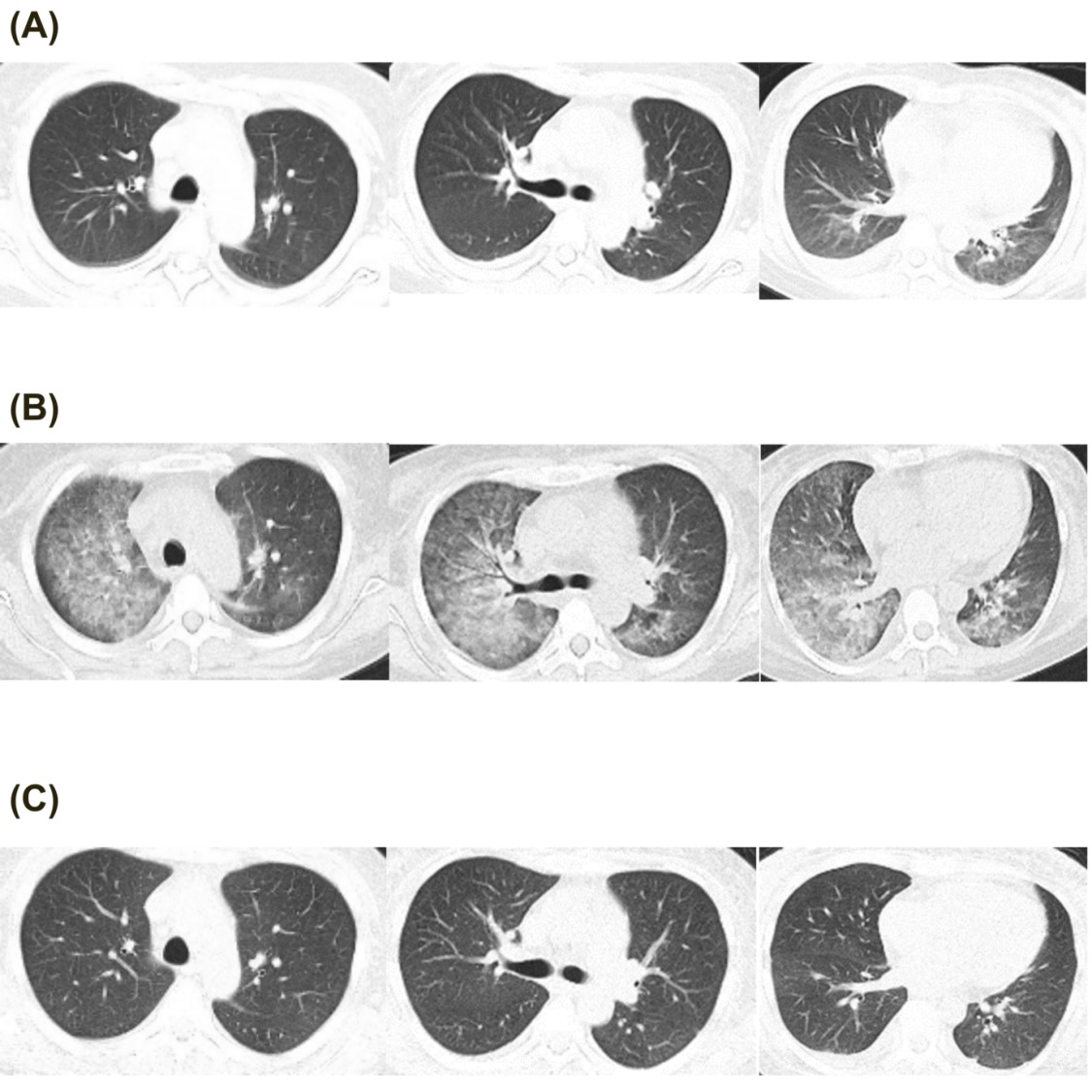
Figure 1: Chest CT images. (A). CT at admission. (B). diffused glass ground opacity on CT after one week of admission. (C). infiltration disappear on CT after two week’s admission.
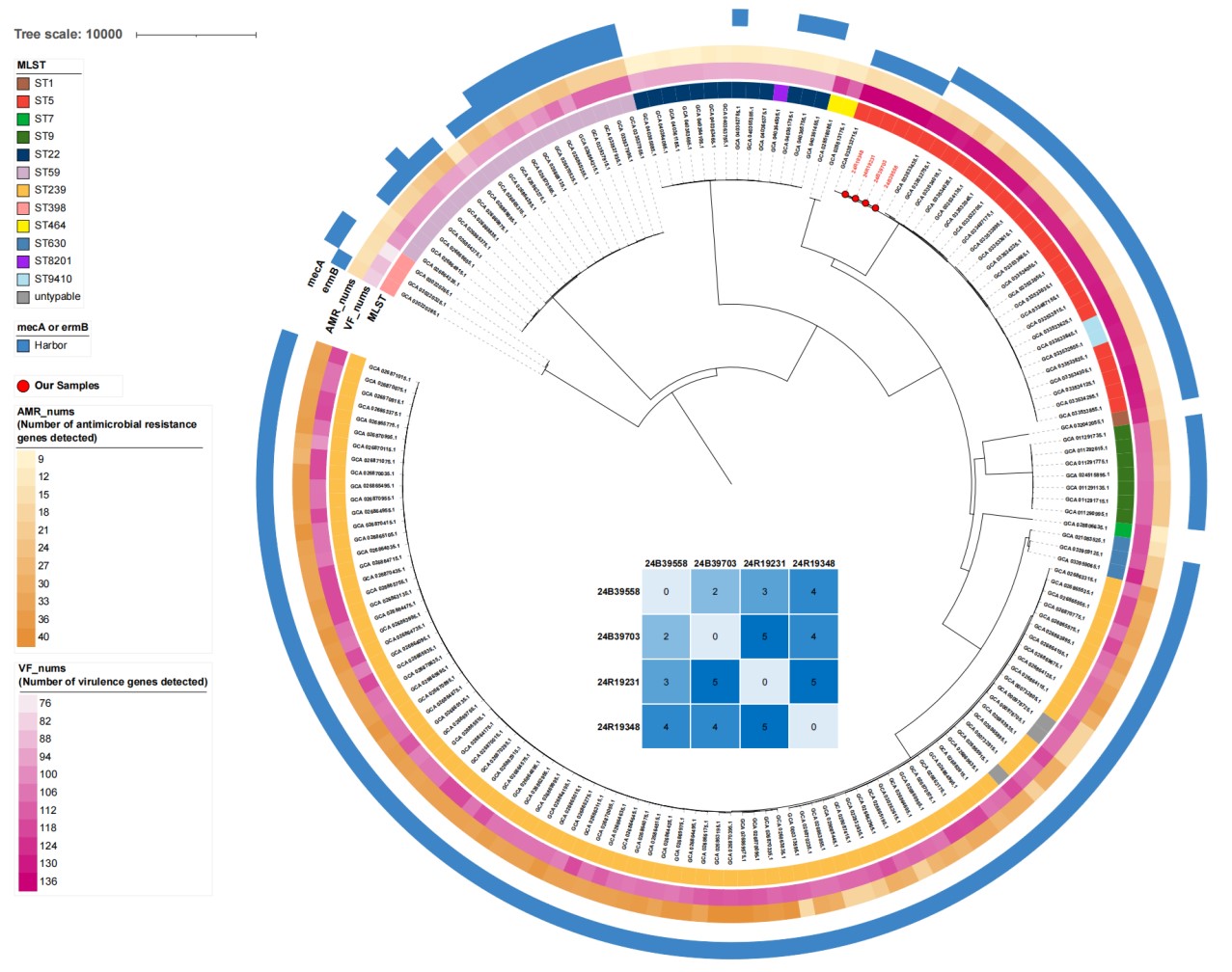
Figure 2: Phylogenetic tree of the 4 isolates with 168 NCBI isolates. Sequence type (MLST), number of antimicrobial resistance genes (AMR_nums) and virulence factors (VF_nums), the presence/absence of mecA and ermB are annotated in the outer rings. The matrix in the center shows the paired core genome SNP differences between the four isolates from the patients, with 24B38558, 24B39703 from blood culture and 24R19231, 24R19348 from respiratory specimens. The 4 isolates are marked with red circles at the tips of the tree.
Discussion
In patients with SLE, bloodstream infections (BSIs) are frequently secondary to infections from the lungs, urinary tract, skin, or catheter-related sites. The pathogens involved are predominantly Gram-negative bacilli, especially Escherichia coli, followed by Gram-positive cocci such as Staphylococcus Aureus (Kogami et al., 2021). In the RELESSER cohort, Staphylococcus Aureus was identified as the second most common pathogen responsible for bacteremia in SLE patients, accounting for 16.7% (19 out of 114 episodes), of which approximately 22% were methicillin-resistant strains (MRSA). Staphylococcus Aureus bacteremia was frequently associated with intravascular catheter use, which was documented in 24.6% of bacteremic episodes, reflecting its known role in catheter-related bloodstream infections.
The chest imaging of bacterial pneumonia typically reveals findings such as lobar or diffuse consolidation, and in some cases cavitation or pleural effusion (Conces, 1998). These imaging signs may overlap with non-infectious lupus manifestations like pulmonary edema or diffuse alveolar hemorrhage, requiring clinical correlation for accurate differentiation (Inui et al., 2023).
This case underscores the critical need to recognise secondary infections in SLE patients with pulmonary edema, particularly those receiving high-dose corticosteroids (Kim et al., 2023). Although pulmonary edema predominated as the explanation for the clinical presentation and imaging findings, the possibility of alveolar-capillary injury facilitating bloodstream invasion by respiratory pathogens must be considered. Such scenarios necessitate heightened vigilance in immunocompromised individuals, where ostensibly non-infectious pulmonary manifestations may still predispose to bacteraemia.
A pivotal diagnostic dilemma arose from discordant findings: sputum cultures identified MSSA despite lung imaging lacking features typical of MSSA pneumonia (Inui et al., 2023). This prompted scrutiny of whether isolates represented colonisation or occult infection. With no alternative bacteraemia sources identified, WGS demonstrated clonal concordance between blood and respiratory isolates, strongly supporting haematogenous dissemination from respiratory colonisation rather than contamination. The alpha–toxin of Staphylococcus Aureus may disrupt endothelial tight junctions, thereby facilitating bacterial invasion into the bloodstream (Hocke et al., 2006).
Conclusion
This report highlights the imperative for systematic microbiological assessment in immunosuppressed patients, even without classical radiological signs of pneumonia. It also illustrates the role of genomic sequencing in elucidating infection pathways, enhancing recognition of occult infections in high-risk populations and informing targeted antimicrobial strategies.
Acknowledgments: NA
Author’s Contribution: Lin Qin and Lina Guo conceptualized this study. Technical support for bioinformatics analysis was provided by Shiyu Chen. Ling Qin and Shiyu Chen wrote and revised the draft article. Chao Li provided instructions on clinical expertise. Ying Ge provided funding supports.
Declaration of Competing Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding: This work was supported by the National High Level Hospital Clinical Research Funding of China (2022-PUMCH-B-043). The patient gave their informed consent for the publication of this study.
References:
Conces DJ Jr. Bacterial pneumonia in immunocompromised patients. J Thorac Imaging 1998; 13: 261-270.
Hocke AC, Temmesfeld-Wollbrueck B, Schmeck B, Berger K, Frisch EM, Witzenrath M, Brell B, Suttorp N, Hippenstiel S. Perturbation of endothelial junction proteins by Staphylococcus aureus alpha-toxin: inhibition of endothelial gap formation by adrenomedullin. Histochem Cell Biol 2006; 126: 305-316.
Inui G, Tomita K, Fukuki M, Touge H, Ikeuchi T, Hisatome I, Yamasaki A. Clinical characteristics for distinguishing between acute cardiogenic pulmonary edema and community-acquired pneumonia in elderly patients: a prospective observational study. Monaldi Arch Chest Dis 2023; 94.
Jolley KA, Maiden MC. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 2010; 11: 595.
Kim MH, Choi SR, Park JK, Lee EY, Lee EB, Park JW. Risk of Bloodstream Infection in Patients with Systemic Lupus Erythematosus Exposed to Prolonged Medium-to-High-Dose Glucocorticoids. Lupus 2023; 32: 625-632.
Kogami M, Abe Y, Shimada Y, Hori S, Tada K, Yamaji K, Tamura N. Bacteremia in systemic lupus erythematosus: Risk factors, clinical and microbiological characteristics, and outcomes in the largest single-center retrospective cohort in Japan. Lupus 2021; 30: 2292-2297.
Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA 2009; 106: 19126-31.
Rúa-Figueroa I, López-Longo FJ, Del Campo V, Galindo-Izquierdo M, Uriarte E, Torre-Cisneros J, Vela P, Tomero E, Narváez J, Olivé A, Freire M, Salgado E, Andreu JL, Martínez-Taboada V, Calvo-Alén J, Hernández-Cruz B, Raya E, Quevedo V, Expósito Pérez L, Fernández-Nebro A, Ibañez M, Pascual-Valls È, Rúa-Figueroa D, Naranjo A, Pego-Reigosa JM. Bacteremia in Systemic Lupus Erythematosus in Patients from a Spanish Registry: Risk Factors, Clinical and Microbiological Characteristics, and Outcomes. J Rheumatol 2020; 47: 234-240.
Schürch AC, Arredondo-Alonso S, Willems RJL, Goering RV. Whole genome sequencing options for bacterial strain typing and epidemiologic analysis based on single nucleotide polymorphism versus gene-by-gene-based approaches. Clin Microbiol Infect 2018; 24: 350-354.
Wang M, Zhang H, Yang X, Li W, Li T, Liu S. Laboratory-confirmed bloodstream infection in systemic lupus erythematosus: Risk profiling and short-term mortality. Lupus 2020; 29: 1520-1527.
Xuan Y, Wang J, Yuan Y, Zhao X, Zuo F, Liu S, Wan L. Risk factors for infections in systemic lupus erythematosus: a meta-analysis. Immunol Res 2025; 73: 103.
|


