FRONTIERS IN MEDICAL CASE REPORTS - Volume 6; Issue 6, (Nov-Dec, 2025)
Pages: 01-15
Print Article
Download XML Download PDF
Evaluation of Weight Loss and Comorbidities Improvements in Morbid Obese and Super Obese Patients After Laparoscopic Roux-en-Y Gastric Bypass: A Ten-Year Single Center Follow-Up
Author: Ida Francesca Gallo, Kiara Sejfullai, Chiara Isabella Miligi, Giuseppe Spagnolo, Vincenzo Bruni
Category: Medical Case Reports
Abstract:
Aim: This study aims to evaluate the long-term efficacy and safety of Roux-en-Y gastric bypass (RYGB) in a cohort of patients from a high-volume center, focusing on outcomes over a ten-year-follow-up period. Methods: A retrospective analysis was conducted on patients who underwent laparoscopic RYGB at Belcolle Hospital between January 2010 and December 2013. Data were collected on weight loss, comorbidity resolution, and complications, with patients stratified into morbidly obese (MO) and super-obese (SO) groups. Results: Out of 149 patients, 71 met the ten-year follow-up criteria. At five years, mean body-mass index (BMI) was 27.8 kg/m², with a percentage of excess weight loss (%EWL) of 79.1%. By ten years, mean BMI increased to 31.8 kg/m², with %EWL decreasing to 61.1%. Comorbidity remission rates were high, with 93.7% of patients achieving diabetes remission. Complications included 11 reported events (15.5%), with no significant differences between MO and SO groups. The majority of patients experienced sustained weight loss and resolution of obesity-related comorbidities. Conclusions: RYGB is a safe and effective treatment for both morbidly and super-obese patients, achieving significant weight loss and improvement in comorbidities over a decade. These findings support expanding the indications for bariatric surgery, although further multicenter prospective studies are needed to mitigate biases inherent in single-center evaluations.
Keywords: Roux-en-Y Gastric Bypass, RYGB, Long-Term Outcomes, Super-Obese Patients, Comorbidities Improvements, Diabetes Remission
Full Text:
Introduction
Obesity is a chronic disease and a significant global health issue, defined by a body mass index (BMI) of 30 kg/m² or higher. Over the past few decades, its prevalence has increased across all age groups (NCD-RisC, 2024). This condition severely impacts quality of life, particularly due to its association with various serious and life-threatening comorbidities. Excess weight is a major risk factor for type II diabetes mellitus, hypertension, dyslipidemia, and numerous other cardiovascular diseases (Courcoulas et al., 2024). The reduction in life expectancy caused by obesity is substantial; for instance, a 25-year-old woman with severe obesity may experience a 22% decrease in his expected remaining lifespan compared to a normal-weight individual (Ward et al., 2022). Since its clinical introduction in the 1950s, Metabolic and Bariatric Surgery (MBS) has gained popularity as a treatment for morbid obesity (Velardi et al., 2024), becoming the standard of care. It has been shown to be the most effective treatment for severe obesity, leading to significant and sustainable weight loss, surpassing medical therapies and lifestyle modifications. In 2013, sleeve gastrectomy (SG) became the most performed bariatric procedure worldwide (Angrisani et al., 2024), although Roux-en-Y gastric bypass (RYGB) is regaining traction due to its beneficial effects on metabolic health (Tu et al., 2022). A recent systematic review confirmed RYGB's association with the remission of type II diabetes and other obesity-related diseases (Raza et al., 2023). Given that obesity is a chronic condition, any weight reduction surgery must demonstrate durability over time. However, few studies have reported long-term outcomes of RYGB concerning comorbidities, weight results and complications. This study aims to evaluate the long-term efficacy and safety of RYGB in patients from a high-volume Italian center, particularly focusing on a ten-year follow-up. The efficacy and safety of RYGB remain a matter of debate in patients suffering from superobesity (SO, BMI > 50 kg/m²), although supported by some retrospective studies (Soong et al., 2021; Mantziari et al., 2022). Its effectiveness, in terms of weight loss and metabolic outcomes, is more controversial when comparing the results of this surgical procedure between patients suffering from SO and morbid obesity (MO, BMI 35-50 kg/m²), with some studies documenting subottimal weight loss in the SO group (Mantziari et al., 2022; Eghbali et al., 2022) and other studies (Verras et al., 2023; Arapis et al., 2019) showing similar results in both groups, even in the long-term. To better understand this topic, we also decided to evaluate and compare the effectiveness and safety of RYGB at 10 years in the two subgroups: MO and SO patients.
Methods
Study Design
Data from patients who underwent laparoscopic primary RYGB at Belcolle Hospital in Viterbo, a high-volume center, between January 2010 and December 2013 by a single surgeon were analyzed retrospectively. Patients were followed for at least ten years post-surgery. All patients met the eligibility criteria for surgery based on the national guidelines in effect at that time, which included being aged 18 to 65 years, having a BMI greater than 40 kg/m², or greater than 35 kg/m² with comorbidities, provided there were no contraindications. A preoperative work-up was performed for all patients, which included an esophagogastroduodenoscopy with Helicobacter pylori screening and evaluation by a multidisciplinary team comprising an endocrinologist, a dietitian, and a psychologist.
Outcome Evaluation
The outcomes considered in our study included weight loss, post-operative complications and evolution of obesity related comorbidities. Weight loss was assessed at assigned time intervals (five and ten years) by means of absolute reduction BMI, percent total bodyweight loss (%TBWL) and Excess Weight Loss (%EWL). %EWL is calculated as [(weight loss) / (pre-operative excess weight)] x 100, in which weight loss is found by initial weight -current weight and pre-operative excess weight by: initial weight – ideal weight. The ideal weight is calculated by the Lorentz formula. For men: height [cm] - 100) - ((height - 150)/4); for women: (height - 100) - ((height - 150)/2). The remission of the comorbidities was evaluated by a blood pressure < 120/80 mm Hg without medication in patients which suffered by hypertension; the cessation of Continuous Positive Airway Pressure (CPAP) machine usage in patients with severe Obstructive Sleep Apnea (OSA); absence of typical symptoms in patients with preoperative GastroEsophageal Reflux Disease (GERD). Diabetes remission was considered as complete normalization of fasting glucose levels (< 126 mg/dL) or HbA1C < 6.5% without any medication, whereas diabetes improvement was defined as better control of diabetes with similar treatment, or similar control with reduced treatment. Early and late complications, readmission and reoperations were recorded.
Surgical Technique
A standardized procedure was performed for all patients. Pneumoperitoneum was induced by the insertion of the Veress needle into the left hypochondrium at Palmer's point. During surgery the CO2 pressure values were always maintained less or equal to 14 mmHg. The optical trocar (10-mm) was introduced in left paramedian site about 15 cm from the xiphoid. Under vision, two 10-mm trocars (at the site of the insertion of the Verres needle and in the right hypochondrium) and two 5-mm trocars (in the right and left hypochondrium) were introduced. The operating table was tilted to the reverse Trendelenburg position. A gastric pouch of 20 mL was obtained and anastomosed with a 30 mm linear stapler to an antecolic jejunal loop at 120 cm from the angle of Treitz. Gastrojejunostomy was completed by closing enterotomies with a double layer absorbable running suture. Approximately 100 cm downstream, a mechanical side-to-side jejunojejunostomy was performed with a 45 mm linear stapler, with the enterotomy closure achieved through a running barbed suture. An intraoperative leak test was performed by introducing methylene blue through a naso-gastric tube, then removed. The “double – loop” was accomplished by sectioning the tract between the gastrojejunostomy and the jejunojejunustomy. Alimentary limb was 100 cm and biliopancreatic limb was 120 cm in all procedures, regardless of patient BMI. Mesenteric defects were never closed. In accordance with the Enhanced Recovery After Surgery (ERAS) protocol, no drain or bladder catheter was routinely placed, and all patients started drinking water 3 hours after surgery. Discharge was typically scheduled for the second post-operative day.
Statistical Analysis
Continuous data were expressed as mean and standard deviation and dichotomous data as number and percentage. Statistical comparisons were performed using Student t-test, Mann-Whitney u-test, Fisher test as appropriate. Repeated measures data were analyzed with mixed-effects models and regression analysis was performed to assess correlations between variables and outcomes.
Statistical significance was defined at p values <0.05.
A post hoc power analysis was performed to assess whether our sample size was sufficient to detect the outcomes of interest.
The statistical analysis was conducted with SPSS IBD ver. 26.0.
Results
Population
Between January 2010 and December 2013, a total of 149 patients underwent RYGB at Belcolle Hospital, Viterbo, Italy. Among them, 78 patients had fewer than 10 years of follow-up, so were excluded from our study.
The study population entailed 71 patients who underwent RYGB and attended regular follow-up visits for at-least 10 years. The population was also divided in two group, 24 patients with superobesity (SO; BMI>50 kg/m²) and 47 patients with morbid obesity (MO; BMI 35-50 kg/m²). Demographic characteristics and comorbidities of all patients are summarized in Table 1.
The mean age of the patients at the time of surgery was 43 ± 10.4 years (range 21–67). The cohort was mainly female (87.3% vs 12.7% male) with a preoperative mean BMI of 47.4 ± 8.2 kg/m² (range 34.9–65.7). Thirty patients were affected by hypertension prior to surgery (42.3%), twenty patients (28.1%) exhibited typical symptoms of GERD, sixteen patients (22.5%) suffered from type II diabetes mellitus, thirteen patients (18.3%) had hyperlipidemia, and seven patients (9.8%) used a CPAP device for obstructive sleep apnea. None had undergone previous bariatric surgery. No statistically significant differences were observed between the two subgroups (MO and SO) in comorbidities’ incidence at baseline, although preoperative BMI was significantly higher in the SO group (57 ± 5 vs 42.5 ± 4.1 kg/m², p-value <0.001).
Table 1: Baseline demographic characteristics and comorbidities.
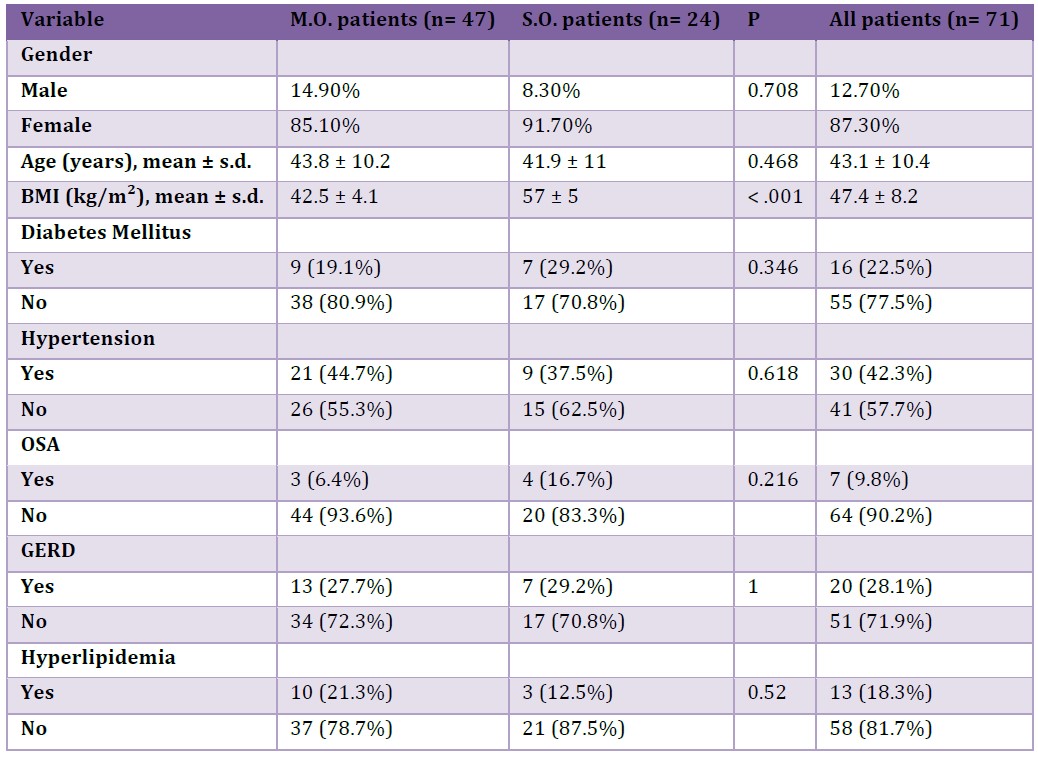
OSA: Obstructive Sleep Apnea, GERD: Gastroesophageal Reflux Disease, BMI: body mass index, SO: superobesity, MO: morbid obesity
Weight Loss Outcomes
At 5 years of follow up, mean BMI was 27.8 ± 4.5 kg/m², with a mean %EWL of 79.1 ± 16.9%. At 10 years the overall BMI was slightly higher (31.8 ± 5.7 kg/m²) but the difference from preoperative value rimained significant (p-value is <0.001); %EWL reduced to 61.1 ± 23.4% 10 years after surgery. The same trend was observed in each subgroup (Graph 1). Overall %TBWL was 42.1% five years after surgery and 33.7% at ten years follow-up (Graph 2) with a significant reduction in SO group compared with MO group (p<0.05). Mixed-effects models revealed that time from surgery was a significant predictor for changes in BMI, %EWL, and %TBWL (p < 0.05 for all masures), indicating that all these variables showed significant changes over the study period. In contrast, SO group was a significant predictor only for %TBWL (p = 0.03), suggesting that the group classification had a significant effect on total body weight loss, but not on the changes in BMI or %EWL over time.
The regression analysis revealed that preoperative BMI was a significant predictor of %TBWL > 20%. The coefficient for BMI was 0.05 (95% CI: 0.02 to 0.08), indicating that for each unit increase in BMI, the odds of achieving %TBWL > 20% increased by approximately 5%. This result was statistically significant (z = 2.5, p = 0.01), as determined by a logistic regression model. In contrast, age and sex were not found to be significant predictors of %TBWL > 20% (p > 0.05 for both variables).
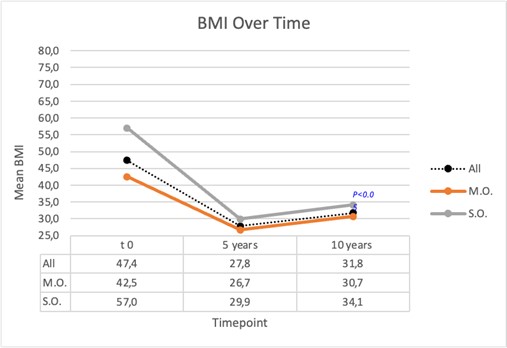
Graph 1: BMI trend over time (mean for all patient and for MO and SO group 5 and 10 years after surgery).
P<0.05 indicates a statistically significant difference between MO and SO group.
Graph 2: %TBWL (mean for all patient and for MO and SO group 5 and 10 years after surgery).
P<0.05 indicates a statistically significant difference between MO and SO group.
Comorbidities
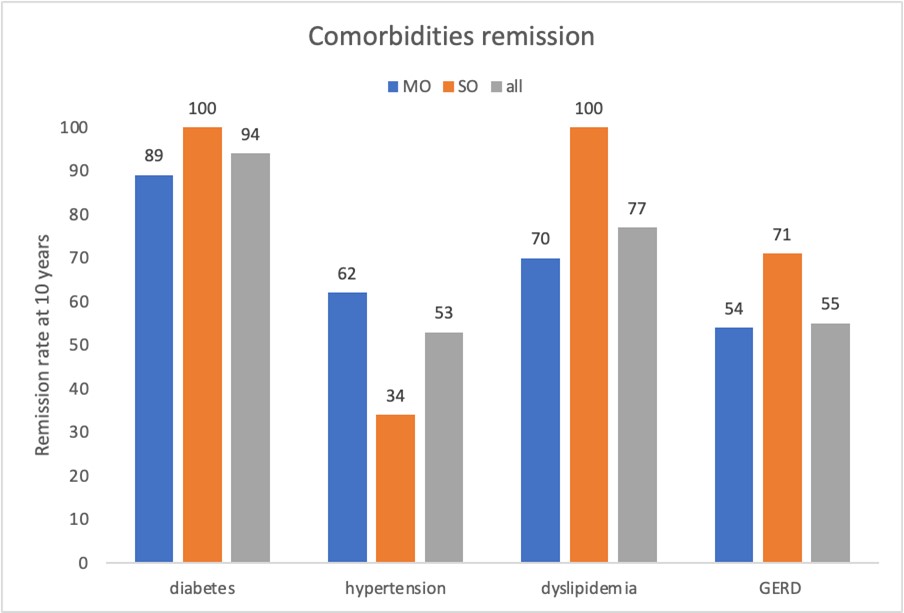
All the evaluated comorbidities, after 10 years follow-up, mainly improved. Among all the patients affected by type II diabetes, 93.7% had remission (100% in the SO group and 88.9% in the MO group, with a Fisher exact test statistic value = 1, not significant at p < .05), which was sustained at the 10-year follow-up. None of the patients with OSAS used a C-PAP device after weight loss surgery. After 10 years, 53,3%, 76,9% and 55% of patients who suffered by hypertension, dyslipidemia, and GERD respectively, resolved their condition (see remission rate for MO and SO group in the graph 3 below); Fisher exact test statistic showed no significant differences at p < 0.05 for each variable).
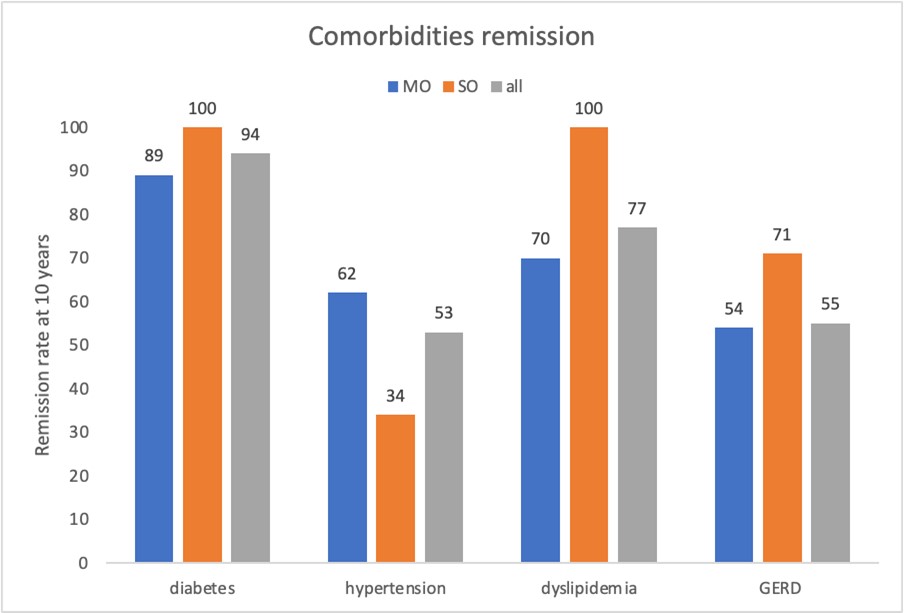
Graph 3: Comorbidities % remission rate over time for all patient and for MO and SO group 10 years after surgery.
Complications
Postoperative complications were classified into early complications (? 90 days after surgery) and late complications (> 90 days postoperative). A total of 11 complications (15.5%) were reported, with 8 occurring in the morbid obesity (MO) group and 3 in the superobesity (SO) group (17% vs. 12.5%, respectively; Fisher's exact test statistic value = 0.739, not significant at p < 0.05). Among these, three complications (4.2%) were classified as early, all of which were gastrojejunostomy leaks (2 in the MO group and 1 in the SO group). Delayed complications totaled 8 (11.2%) and included five internal hernia (7%), one incisional hernia (1.4%), and two cases of chronic anemia requiring repeated iron infusions (2.8%). There were no thromboembolic events or mortality reported (Graph 4-5). Age, sex and preoperative comorbidities were not found to be significant predictors of complications in our series.
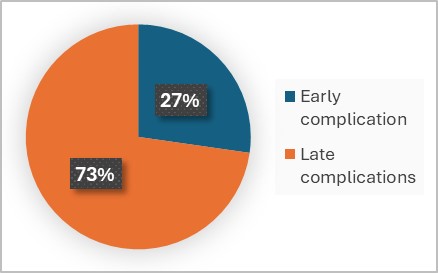
Graph 4: Percentage of early and late complications above all.
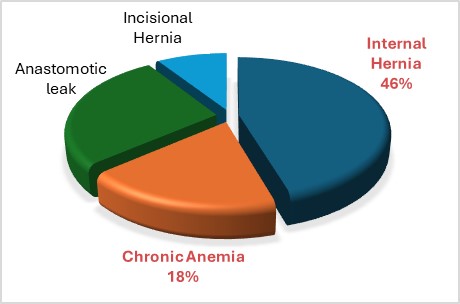
Graph 5: Percentage of kind of complication above all.
Discussion
Our study provides one of the few long-term evaluations of RYGB safety and efficacy, with a complete 10-year follow-up from a single high-volume center, in both morbidly obese (MO) and super obese (SO) subgroups. Patients achieved and maintained clinically significant weight loss, with %TBWL exceeding the 20% threshold considered a marker of success. Additionally, the study shows high long-term remission rates of obesity-related comorbidities, alongside a low rate of complications and no mortality. These results support RYGB as an effective metabolic and bariatric treatment, even in patients with BMI > 50 kg/m², challenging assumptions that these individuals may face higher risks and few benefits.
The weight loss observed in our study aligns with findings from previous research, such as the study by Verras, et al. (2023), which reported that patients undergoing RYGB lost 37.6% of their baseline weight at the 10-year mark. In our cohort, patients lost an average of 42.1% of their total body weight five years post-surgery, maintaining a 33.7% total body weight loss at the 10-year follow-up.
Despite the results showing the long-term effectiveness of the intervention, a small percentage of patients experienced weight regain (9/71, 12.6 %). Although investigating the causes of any weight regain (lifestyle, eating habits, post-operative metabolic adaptation, etc.) is a key-point in patient management, it was not the target of this study; briefly, patients with a BMI rebound in our center are evaluated by the multidisciplinary team (surgeon, psychologist, dietician, endocrinologist) to find the underlying cause and set the most appropriate treatment.
The main concerns regarding super obese patients are that surgical weight loss outcomes, including those of gastric bypass, may be less favorable and associated with higher surgical risks than in non-super obese patients. This is largely due to a higher prevalence of obesity-related comorbidities and the technical challenges posed by central obesity. Consequently, the use of metabolic and bariatric surgery in this population remains debated, often perceived as less effective and safe than in patients with a BMI below 50 kg/m² (Arapis et al., 2019).
Critics of surgical effectiveness often argue that many super obese patients do not achieve a non-obese status post-surgery. Such criticisms set unrealistic expectations that patients should reach a BMI below 30 following MBS. These expectations can be dangerous; the primary benefit of surgery lies in the weight lost rather than the final BMI. Studies on long-term weight loss have consistently shown that the average weight loss with RYGB is approximately 25–30% of total body weight (Nedelcu et al., 2023), which typically does not result in patients becoming non-obese (i.e., a BMI below 30) but is associated with significant health and well-being improvements.
Regarding this endpoint of our study, a significant challenge is that an ideal metric for quantifying the effectiveness of weight loss after MBS has not yet been established, resulting in various definitions within the literature and a lack of clear consensus.
This debate involves numerous organizations, including the American Medical Association (AMA), which has recently recommended using BMI in conjunction with at least one additional metric to classify obesity. BMI remains a useful parameter for classifying obesity and identifying patients who are more likely to develop obesity-related diseases, but it has several limitations that reduce its effectiveness. The most significant limitation is that it is a relatively poor indicator of metabolic status. Individuals with the same BMI do not necessarily have the same degree of fat accumulation or distribution, leading to different obesity phenotypes (Pantelis, 2024) and highly varied metabolic consequences. It is possible that, in the future, new multiparametric classification methods (such as the Edmonton Obesity Staging System) will be adopted to redefine the indications and categories of patients eligible for MBS. All these challenges complicate the standardization of weight loss outcomes and hinder the interpretation and comparison of results across studies.
To address this issue, we calculated all major parameters used to define weight loss, facilitating comparisons with as many studies as possible. Many researchers consider the percentage of total body weight loss (%TBWL) relative to preoperative weight as the most accurate metric for assessing weight loss. This parameter is less influenced by baseline weight, remaining relatively consistent across different obesity classes, whereas metrics like %EWL are significantly affected by initial BMI and may lead to misleading conclusions about the efficacy of weight loss (Shahwan et al., 2022). A recent position statement from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) regarding therapeutic options for addressing weight recurrence and obesity-related complications after metabolic and bariatric surgery defined optimal clinical response as %TWL ? 20% and/or improvement in obesity-related complications (Haddad et al., 2024).
In the absence of statistically significant differences between the two subgroups regarding the incidence of comorbidities at baseline, our results indicate favorable outcomes, with a percentage of total body weight loss (%TBWL) exceeding 20% in both groups. Notably, the super obese group showed a greater response than the morbidly obese group, achieving %TBWL of 47.2% compared to 37% at five years, and 39.9% compared to 27.5% at ten years post-surgery (p<0.05). This contrasts with studies suggesting a reduced weight loss effect and higher rates of weight regain in the super obese population8. For instance, the study by Aftab, et al. (2014) reported greater weight loss in the super obese group (30% vs. 26% total body weight loss, p=0.008), without significant differences in %EWL. Our study showed no significant differences in %EWL between the two groups (p>0.05, see Graph 6).
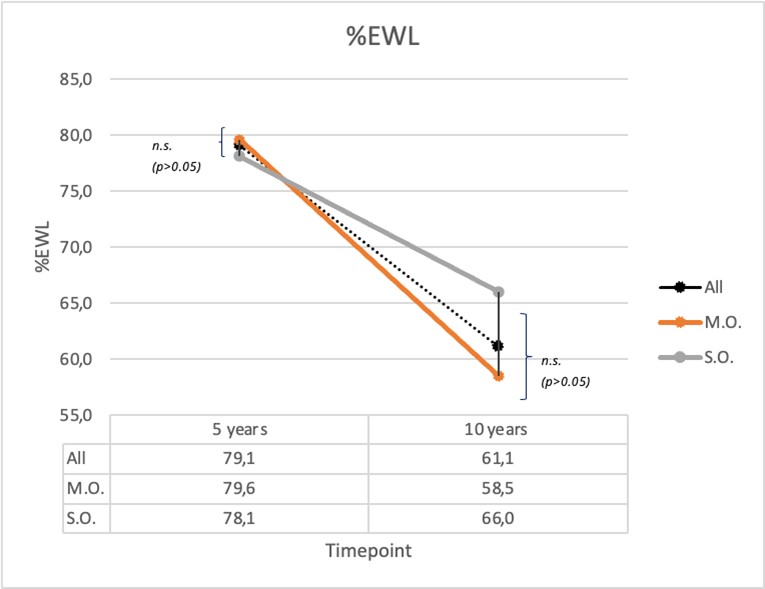
Graph 6: %EWL (mean for all patient and for MO and SO group 5 and 10 years after surgery).
P>0.05 indicates no significant difference between MO and SO group.
Our findings suggest that super obese patients should not be excluded from RYGB indications based solely on weight loss outcomes. Additionally, preoperative BMI did not appear to influence weight loss, emphasizing the importance of considering other preoperative conditions to predict a patient’s success after MBS.
Despite the perception of lesser weight loss at least when reported as %EWL, most studies report relatively equally significant restoration of metabolic comorbidities and quality of life (QOL) in morbid and super obese patients.
Given its role in metabolic regulation, the gastrointestinal tract constitutes a meaningful target to manage type II diabetes mellitus. Since 2016, with the DSS-II guidelines (Rubino et al., 2016), metabolic surgery was included in the treatment algorithm for patients with BMI above 35 kg/m2 and type II diabetes mellitus inadequately controlled by lifestyle and optimal medical therapy. Recently, the new Italian and IFSO guidelines (SICOB guidelines, 2023; Eisenberg et al., 2022), extended this indication also to patients with BMI between 30 and 34.9 kg/m2. Courcoulas, et al. (2024) pooled analysis reinforces the view that adding metabolic surgery, particularly RYGB, to the best medical treatment is a good option for the management of uncontrolled T2D in patients with a BMI ? 30 kg/m2, showing, in conclusion, strong evidence that RYGB improves metabolic outcomes. Our study is in line with these results, with a diabetes rate remission of 93.7%. Our diabetes remission rate is remarkable, higher than in the literature. This data could be due to the small number of patients and the duration of the disease at the time of surgery given that, as is known, the remission rate is also linked to the time of onset of the disease.
Our data also shows that RYGB effectively controls not only T2D but the major three endpoints of the metabolic syndrome (hypertension, hyperglycemia, dyslipidemia) over 10 years after surgery. These comorbidities have a chronic course exactly like obesity, so it was shown how removing one of the main chronic risk factors it is possible to achieve a remission of them, unavoidably always in association with an improvement in lifestyle, thus preventing chronic recurrence. Twenty patients in our study experienced typical GERD symptoms prior to surgery and required daily treatment with proton pump inhibitors. Our findings support the recommendation of Roux-en-Y gastric bypass (RYGB) as the preferred surgical technique in such cases, as it effectively resolves symptoms in most patients, unlike sleeve gastrectomy. A study by Biter, et al. (2024) found a high incidence of reflux five years after sleeve gastrectomy, with 16% of their patients reporting GERD symptoms (vs 4% in Roux-en-Y gastric bypass patients) requiring medical therapy or revisional surgery.
In our study, none of the examined comorbidities showed a significantly greater reduction in either group, further confirming the efficacy of RYGB, including in super obese patients.
Regarding the complications associated with MBS and concerns for patients with a BMI greater than 50 kg/m²-such as increased visceral adipose tissue and a thicker abdominal wall, which could complicate surgery-we found no increase in operative morbidity. The complication rates were remarkably similar in both groups, and no deaths occurred during the 10-year follow-up period. Among the late complications, we observed five internal hernias requiring surgical intervention (7%), one incisional hernia (1.4%), and two cases of chronic anemia necessitating repeated iron infusions (2.8%).
Internal hernia is a potential complication following RYGB, with an average incidence of 2.5% after laparoscopic procedures. Clinical manifestations of internal hernia can vary, ranging from mild, intermittent abdominal pain to life-threatening conditions such as small bowel obstruction and ischemia (Kollmann et al., 2023). This complication arises from the creation of inter-mesenteric spaces during surgery. Potential sites for herniation include the mesenteric defect at the jejuno-ileal anastomosis and the space between the transverse mesocolon and the mesentery of the gastro-jejunal anastomosis (Petersen's space). The "double loop" technique employed in our study does not require the opening of the mesentery during the construction of the Roux limb, thereby reducing the risk of internal hernia formation at this site. At the time of the RYGB procedures considered, the mesenteric windows were never closed. As highlighted in a recent study (Gallo et al., 2025) reviewing bypass procedures performed in our center, although in our series IH incidence was lower than reported in many other studies, it remains the most frequent complications following RYGB. This consideration suggests that mesenteric defect should be closed during primary surgery, although standardized guidelines on the optimal surgical technique for closure are still lacking.
Regarding malnutrition, previous bariatric procedures, such as biliopancreatic diversion (BPD), have resulted in significant vitamin and macronutrient deficiencies, leading to potentially life-threatening conditions. It is well established that the risk of hypoproteinemia and other deficiencies is considerably lower in patients undergoing Roux-en-Y gastric bypass (RYGB) (Mantziari et al., 2023). The absorption of intestinal nutrients is closely related to the length of the biliary limb; a longer limb can enhance metabolic effects but also increases the risk of malnutrition. In our technique, we consistently utilized a 120 cm biliary limb. To prevent nutrient deficiencies, we prescribed lifelong multivitamin supplementation to all patients. In our study, only two patients (2.8%) developed chronic anemia requiring repeated iron infusions.
In conclusion, our data suggest that RYGB is an effective treatment for the bariatric population, achieving significant weight loss and high rate of weight-related comorbidities’ resolution. Additionally, it has proven to be a safe and effective option not only for morbidly obese patients but also for super obese individuals, thereby expanding the indications for bariatric surgery.
Limitations
Despite the valuable findings provided by this study on the long-term efficacy and safety of laparoscopic RYGB, several limitations should be acknowledged. Firstly, the retrospective design represents a fundamental limitation, since it can lead to selection bias and limits the control of the confounding variables, with the risk of missing or inaccurate data, affecting the reliability and validity of the results. As mentioned before in the manuscript, BMI and weight-based metrics, have inevitable limitations in the description of the body composition and metabolic health, particularly across diverse obesity phenotypes. To address this issue, we calculated all major parameters used to define weight loss, facilitating comparisons with as many studies as possible.
In this study we analyzed the post-operative course focusing on weight regain and complications such as gastrojejunostomy leaks, anemia and internal or abdominal hernias, leaving behind the aspect concerning the psychosocial, behavioral, or dietary factors that could for sure influence the postoperative results.
Although we included all patients who underwent primary laparoscopic RYGB within the specified period and had complete 10-year follow-up, nearly half of the patients (78 out of 149) were excluded due to loss to follow-up. This could introduce bias, as patients with suboptimal outcomes or complications may be more likely to disengage from long-term follow-up.
Furthermore, the overall sample size is limited, particularly when divided into the two subgroups. This may limit the study’s statistical power to detect meaningful differences in secondary outcomes, such as complication rates and comorbidity remission.
Lastly, our findings reflect the experience of a single surgeon at a single high-volume center in Italy. While this leads to procedural consistency, it may limit generalizability, as outcomes may not be replicable in lower-volume centers or with different surgical teams or surgical protocols.
Future prospective, multicenter studies with larger sample sizes are needed to fully validate and extend our findings.
Declarations
Ethical Approval and Consent to Participate: Ethical Approval of the study was obtained (119.25 CET2 cbm). Informed consent was obtained from all participants. The study was performed in accord with the ethical standards of the Declaration of Helsinki.
References:
Aftab H, Risstad H, Søvik TT, Bernklev T, Hewitt S, Kristinsson JA, Mala T. Five-year outcome after gastric bypass for morbid obesity in a Norwegian cohort. Surg Obes Relat Dis 2014; 10: 71-8.
Angrisani L, Santonicola A, Iovino P, Palma R, Kow L, Prager G, Ramos A, Shikora S; Collaborative Study Group for the IFSO Worldwide Survey. IFSO Worldwide Survey 2020-2021: Current Trends for Bariatric and Metabolic Procedures. Obes Surg 2024; 34: 1075-1085.
Arapis K, Macrina N, Kadouch D, Ribeiro Parenti L, Marmuse JP, Hansel B. Outcomes of Roux-en-Y gastric bypass versus sleeve gastrectomy in super-super-obese patients (BMI ?60 kg/m2): 6-year follow-up at a single university. Surg Obes Relat Dis 2019; 15: 23-33.
Biter LU, 't Hart JW, Noordman BJ, Smulders JF, Nienhuijs S, Dunkelgrün M, Zengerink JF, Birnie E, Friskes IA, Mannaerts GH, Apers JA. Long-term effect of sleeve gastrectomy vs Roux-en-Y gastric bypass in people living with severe obesity: a phase III multicentre randomised controlled trial (SleeveBypass). Lancet Reg Health Eur 2024; 38: 100836.
Courcoulas AP, Patti ME, Hu B, Arterburn DE, Simonson DC, Gourash WF, Jakicic JM, Vernon AH, Beck GJ, Schauer PR, Kashyap SR, Aminian A, Cummings DE, Kirwan JP. Long-Term Outcomes of Medical Management vs Bariatric Surgery in Type 2 Diabetes. JAMA 2024; 331: 654-664.
Eghbali F, Bahardoust M, Pazouki A, Barahman G, Tizmaghz A, Hajmohammadi A, Karami R, Hosseini-Baharanchi FS. Predictors for weight loss after Roux-en-Y gastric bypass: the trend and associated factors for weight loss. BMC Surg 2022; 22: 310.
Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RV, De Luca M, Faria SL, Goodpaster KPS, Haddad A, Himpens JM, Kow L, Kurian M, Loi K, Mahawar K, Nimeri A, O'Kane M, Papasavas PK, Ponce J, Pratt JSA, Rogers AM, Steele KE, Suter M, Kothari SN. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg Obes Relat Dis 2022; 18: 1345-1356.
Gallo IF, Marrelli M, Miligi CI, Spagnolo G, Bruni V. Incidence and management of internal hernia after laparoscopic Roux-en-Y gastric bypass without preventive closure of mesenteric defects: a single-center retrospective study. Updates Surg 2025; 77: 2001-2006.
Haddad A, Suter M, Greve JW, Shikora S, Prager G, Dayyeh BA, Galvao M, Grothe K, Herrera M, Kow L, Le Roux C, O'Kane M, Parmar C, Quadros LG, Ramos A, Vidal J, Cohen RV. Therapeutic Options for Recurrence of Weight and Obesity Related Complications After Metabolic and Bariatric Surgery: An IFSO Position Statement. Obes Surg 2024; 34: 3944-3962.
Kollmann L, Lock JF, Kollmann C, Vladimirov M, Germer CT, Seyfried F. Surgical treatment of internal hernia after Roux-en-Y gastric bypass - impact of institutional standards and surgical approach. Langenbecks Arch Surg 2023; 408: 318.
Mantziari S, Abboretti F, Favre L, Thomopoulos T, Barigou M, Demartines N, Suter M. Protein malnutrition after Roux-en-Y gastric bypass: a challenging case and scoping review of the literature. Surg Obes Relat Dis 2023; 19: 746-754.
Mantziari S, Thomopoulos T, Abboretti F, Gaspar-Figueiredo S, Dayer A, Demartines N, Suter M. Long-term weight loss and metabolic benefit from Roux-en-Y gastric bypass in patients with superobesity. BJS Open 2022; 6: zrac145.
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024; 403: 1027-1050.
Nedelcu M, Laclau-Lacrouts M, Najah H, Carandina S, Monsaingeon M, Pupier E, Collet D, Gatta-Cherifi B, Gronnier C. Long-Term Results After Bariatric Surgery in Super-Super-Obese Patients. J Laparoendosc Adv Surg Tech A 2023; 33: 536-541.
Pantelis AG. Standardizing outcomes in metabolic bariatric surgery-more than meets the eye, less than counts the scale. Metabolism and Target Organ Damage 2024; 4: N-A.
Raza MM, Njideaka-Kevin T, Polo J, Azimuddin K. Long-Term Outcomes of Bariatric Surgery: A Systematic Review. Cureus 2023; 15: e39638.
Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH, Amiel SA, Kaplan LM, Taroncher-Oldenburg G, Cummings DE; Delegates of the 2nd Diabetes Surgery Summit. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care 2016; 39: 861-877.
Shahwan S, Oochit K, Campbell E, Kourounis G. Reporting of weight loss outcomes in bariatric surgery following introduction of 2015 ASMBS guidelines. Surg Obes Relat Dis 2022; 18: 1195-1198.
SICOB guidelines, 2023 September.
[URL: https://www.sicob.org/00_materiali/Linee_Guida_SICOB_2023.pdf]
Soong TC, Lee MH, Lee WJ, Almalki OM, Chen JC, Wu CC, Chen SC. Long-Term Efficacy of Bariatric Surgery for the Treatment of Super-Obesity: Comparison of SG, RYGB, and OAGB. Obes Surg 2021; 31: 3391-3399.
Tu J, Wang Y, Jin L, Huang W. Bile acids, gut microbiota and metabolic surgery. Front Endocrinol (Lausanne) 2022; 13: 929530.
Velardi AM, Anoldo P, Nigro S, Navarra G. Advancements in Bariatric Surgery: A Comparative Review of Laparoscopic and Robotic Techniques. J Pers Med 2024; 14: 151.
Verras GI, Mulita F, Pouwels S, Parmar C, Drakos N, Bouchagier K, Kaplanis C, Skroubis G. Outcomes at 10-Year Follow-Up after Roux-en-Y Gastric Bypass, Biliopancreatic Diversion, and Sleeve Gastrectomy. J Clin Med 2023; 12: 4973.
Ward ZJ, Willett WC, Hu FB, Pacheco LS, Long MW, Gortmaker SL. Excess mortality associated with elevated body weight in the USA by state and demographic subgroup: A modelling study. EClinicalMedicine 2022; 48: 101429.
|







